send link to app
Common Terminology Criteria For Adverse Events (4.03)
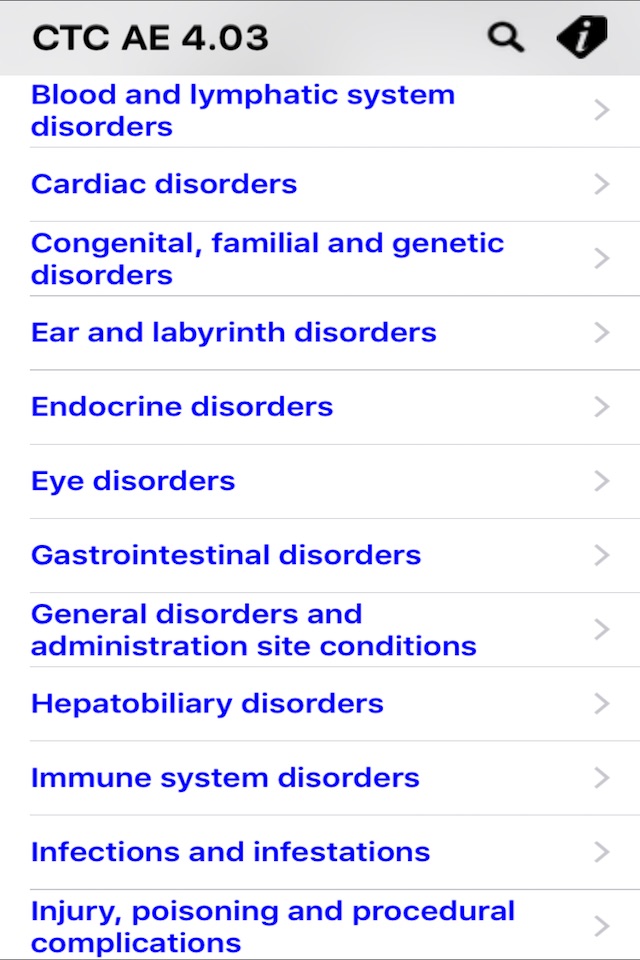
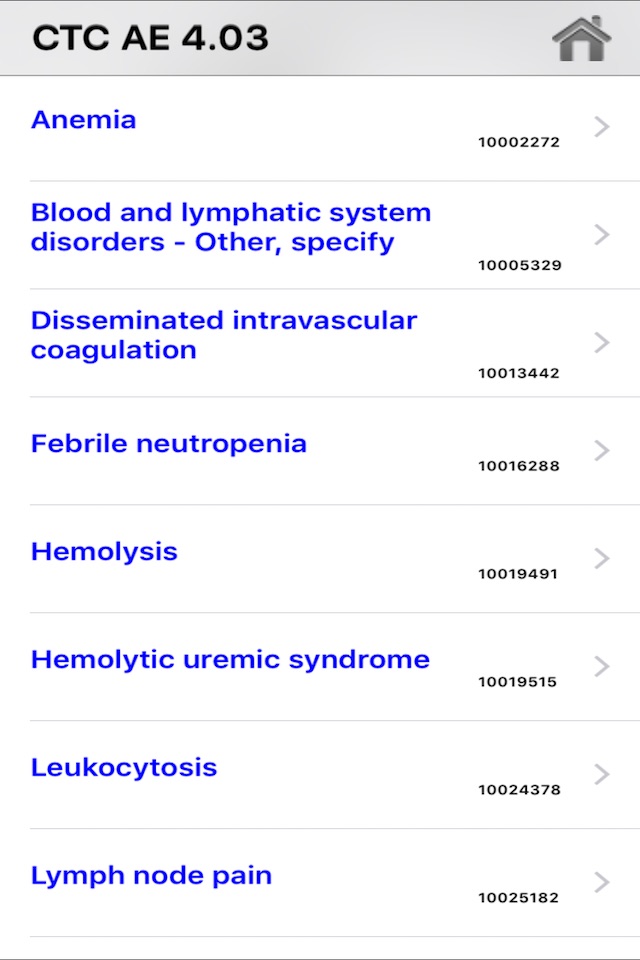
This software allows you the selection of the correct descriptive terminology for reporting adverse events. For each AE (Adverse Event) term, the relative MedDRA (Medical Dictionary for Regulatory Activities) term definition and codification, in addition to a grading severity scale (from 1 to 5) are provided.
A powerful search engine, integrated in the software, allows you to quickly display all information related to a specific AE.